Thermal Conductors and Insulators
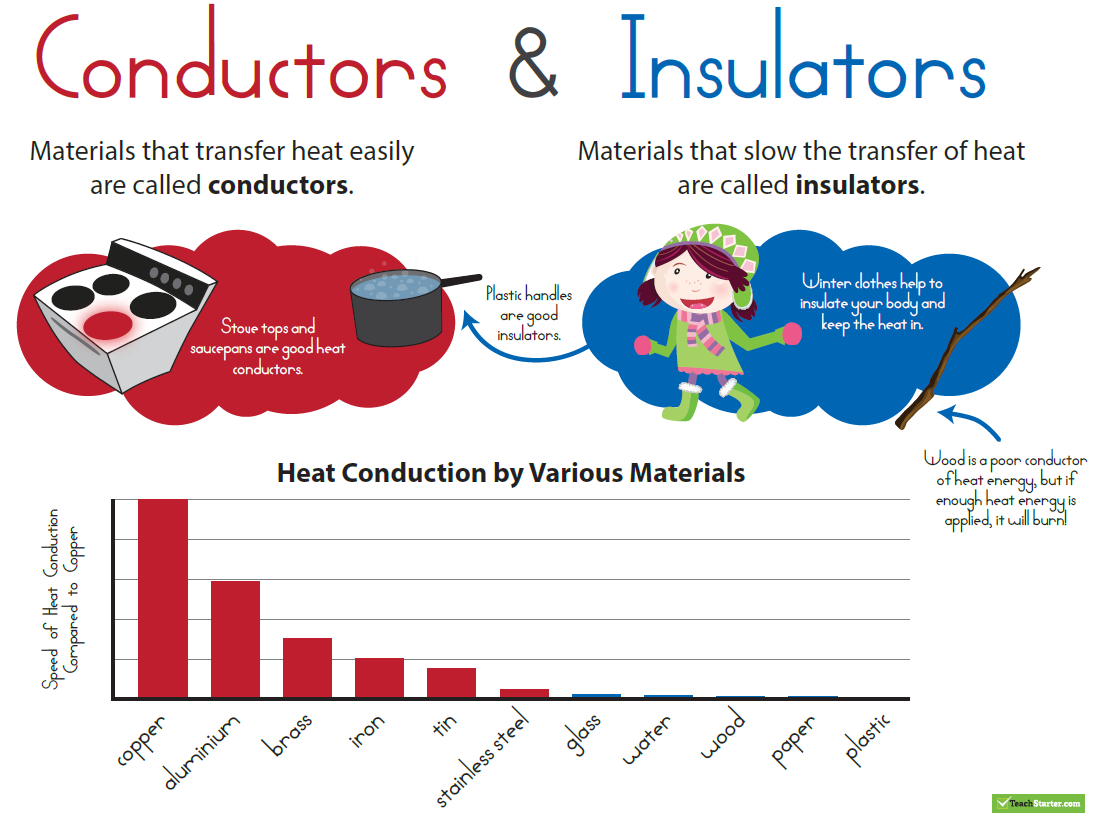
Everything has the ability to be a conductor of heat, though some act as better conductors than others.
Do you like toast? Did you ever look inside a toaster while it’s toasting bread? When you push down the lever to turn on the toaster, the metal heating element inside starts to glow orange or red almost instantly. You can see the glowing heating element inside this yellow toaster. The glowing metal shows that the heating element has become very hot. It gets hot so quickly because metals are good conductors of thermal energy.
Thermal Conductors
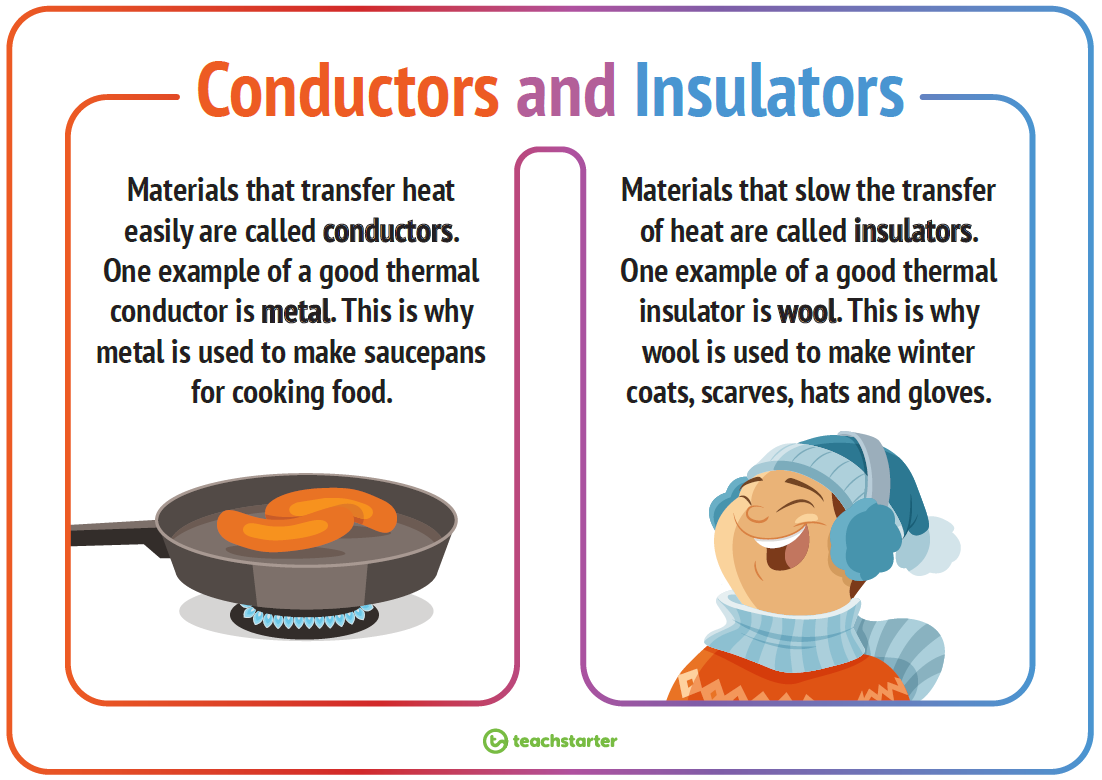
Conduction is the transfer of thermal energy between particles of matter that are touching. Thermal conduction occurs when particles of warmer matter bump into particles of cooler matter and transfer some of their thermal energy to the cooler particles. Conduction is usually faster in certain solids and liquids than in gases. Materials that are good conductors of thermal energy are called thermal conductors. Metals are especially good thermal conductors because they have freely moving electrons that can transfer thermal energy quickly and easily.
Besides the heating element inside a toaster, another example of a thermal conductor is a metal radiator, like the one in the Figure below. When hot water flows through the coils of the radiator, the metal quickly heats up by conduction and then radiates thermal energy into the surrounding air.6aa300Besides the heating element inside a toaster, another example of a thermal conductor is a metal radiator, like the one in the Figure below. When hot water flows through the coils of the radiator, the metal quickly heats up by conduction and then radiates thermal energy into the surrounding air.

Thermal Conductors and Insulators ( Read ) | Physics | CK-12 Foundation
Q: Thermal conductors have many uses, but sometimes it’s important to prevent the transfer of thermal energy. Can you think of an example?
A: One example is staying warm on a cold day. You will stay warmer if you can prevent the transfer of your own thermal energy to the outside air.
Thermal Insulators
One way to retain your own thermal energy on a cold day is to wear clothes that trap air. That’s because air, like other gases, is a poor conductor of thermal energy. The particles of gases are relatively far apart, so they don’t bump into each other or into other things as often as the more closely spaced particles of liquids or solids. Therefore, particles of gases have fewer opportunities to transfer thermal energy. Materials that are poor thermal conductors are called thermal insulators. Down-filled snowsuits, like those in the Figure below, are good thermal insulators because their feather filling traps a lot of air.

Another example of a thermal insulator is pictured in the Figure below. The picture shows fluffy pink insulation inside the attic of a home. Like the down filling in a snowsuit, the insulation traps a lot of air. The insulation helps to prevent the transfer of thermal energy into the house on hot days and out of the house on cold days. Other materials that are thermal insulators include plastic and wood. That’s why pot handles and cooking utensils are often made of these materials. Notice that the outside of the toaster pictured in the opening image is made of plastic. The plastic casing helps prevent the transfer of thermal energy from the heating element inside to the outer surface of the toaster where it could cause burns.

Q: Thermal insulators have many practical uses besides the uses mentioned above. Can you think of others?
A: Thermal insulators are often used to keep food or drinks hot or cold. For example, Styrofoam® coolers and thermos containers are used for these purposes.